Liger Medical is building the railroads of cervical cancer elimination in India with our cutting edge screening and treatment products
About Us
Finding the Best Fit Products for You
We are building the infrastructure for an India free of cervical cancer. We are fuelled by our charter to eliminate cervical cancer, which is currently the second most common cancer affecting Indian women.
Our vision is simple: an India where women aren’t torn apart from their families due to cervical cancer.
We want to enable access to cervical cancer care in the most rural parts of India.

Lightweight, portable,
battery operated

Audio and visual cues for patient safety

Easy to use

Safe and effective



Our products are novel and designed to be effective and easy to use.
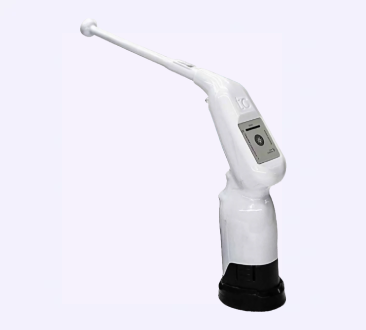
HTU-110

ESU-110
Treat cervical cancer safely and effectively with our battery operated, lightweight electrosurgical unit ESU-110. ESU-110 is the only portable and battery operated device available on the market, designed to perform LEEP/LLETZ easily and effectively.

IRIS
Screen, document, diagnose and treat cervical cancer safely and effectively with IRIS- our turnkey solution for cervical cancer elimination.
case use video

Cervical Cancer
The 4th most common
form of cancer worldwide
300,000 women
succumb every year
128,000 cases in India with 78000 fatalities every year
There are challenges -

The disease is highly prevalent in remote & rural areas which lack equipment to treat precancerous lesions effectively

Social stigma fuelled by a lack of awareness about primary & secondary prevention methods adds to the problem

60% women screened for PAP or HPV do not return for treatment (global figure)
Clearly, there is an acute need for single point of care solutions that are highly resilient to low resource settings

The World Health Organization has recommended a 90-70-90 elimination target to be met by 2030
90% of girls fully vaccinated with HPV vaccine by age 15 years
Only 15% girls vaccinated in 2020 (worldwide)
70% women screened by 35 years of age & again by 45 years of age
Lifetime screening in India hovers at 2% , as per NFHSV.
90% women identified with cervical disease to receive treatment
Only 40% of identified cases being treated (worldwide)
Success Stories
Groesbeck P. Parham, MD, Professor, Honorary Consultant, University of Zambia
I just used the tc for CIN2-3, I only had to do one cycle, it was great, so smooth. My patient asked me to thank you for making the device so much better than cryo!!!
Dr. Heather Jackson,
Family Practice, Salt Lake City,
Utah, USA
“The procedure went well. The 19 mm probe works well and I used it for 2-3 application as the cervix was bulky. The heating time, timer, etc. works well and cervix appears exactly how it appears after treatment with cold coagulation.”
Smita Joshi,
MD, Hirabai Hospital Pune, India
Cervical Cancer Organizations
















Liger Medical

Present in 50+ countries

Large distribution network

Reliable & Cost Effective
healthcare devices

- Cambodia
- Venezuela
- Bangladesh
- Nepal
- China
- Mongolia
- USA
- Ecuador
- Ethiopia
- Congo
- Eswatini
- EI Salvador
- Australia
- Bolivia
- Tanzania
- Colombia
- India
- Burkina Faso
- Belize
- South Africa
- Malaysia
- Senegal
- Malawi
- Papua New Guinea
- Peru
- Tanzania
- Philippines
- Rwanda
- Sierra Leone
- Mexico
- Guatemala
- Aruba
- Nicaragua
- Gambia
- Kenya
- Ghana
- Nigeria
- Mozambique
- Mali
- Zambia
- Switzerland
- Nicaragua
- Honduras
- Haiti
- Thailand
- Cameroon
- Uganda
- Tuvalu
- Singapore
- Costa Rica
- France
- Mauritania
- Burundi
- Vietnam
- Botswana
Scientific studies

Overview of thermal ablation devices for treating precancerous cervical lesions in low resource settings

Thermal ablation versus cryotherapy or loop excision to treat women positive for cervical precancer on visual
Background Cryotherapy is standard practice for treating patients with cervical precancer

Thermal ablation of premalignant lesions of uterine cervix using a portable battery-driven device

The new england journal of medicine
In October 1999, we began to measure the effect of a single round of screening by testing for human




